Synthesis of 3-[(N-carboalkoxy)ethylamino]-indazole-dione derivatives and their biological activities on human liver carbonyl reductase.
Solomon Berhe, Andrew Slupe, Choice Luster, Henry A Charlier, Don L Warner, Leon H Zalkow, Edward M Burgess, Nkechi M Enwerem, Oladapo Bakare
Index: Bioorg. Med. Chem. 18(1) , 134-41, (2010)
Full Text: HTML
Abstract
A series of indazole-dione derivatives were synthesized by the 1,3-dipolar cycloaddition reaction of appropriate substituted benzoquinones or naphthoquinones and N-carboalkoxyamino diazopropane derivatives. These compounds were evaluated for their effects on human carbonyl reductase. Several of the analogs were found to serve as substrates for carbonyl reductase with a wide range of catalytic efficiencies, while four analogs display inhibitory activities with IC(50) values ranging from 3-5 microM. Two of the inhibitors were studied in greater detail and were found to be noncompetitive inhibitors against both NADPH and menadione with K(I) values ranging between 2 and 11 microM. Computational studies suggest that conformation of the compounds may determine whether the indazole-diones bind productively to yield product or nonproductively to inhibit the enzyme.Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
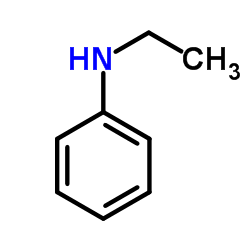 |
N-Ethylaniline
CAS:103-69-5 |
C8H11N |
|
cIEF for rapid pKa determination of small molecules: a proof...
2014-10-15 [Eur. J. Pharm. Sci. 63 , 14-21, (2014)] |
|
Convenient QSAR model for predicting the complexation of str...
2009-01-01 [Bioorg. Med. Chem. 17 , 896-904, (2009)] |
|
Spectroscopic studies of molecular interactions involving 2,...
2007-01-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(1) , 94-101, (2007)] |
|
Interference with analysis of amphetamine in blood by N-ethy...
1988-01-01 [J. Anal. Toxicol. 12(3) , 147-9, (1988)] |
|
Catalyst-free aqueous multicomponent domino reactions from f...
[Green Chem. 11(12) , 1968-72, (2009)] |