| Structure | Name/CAS No. | Articles |
|---|---|---|
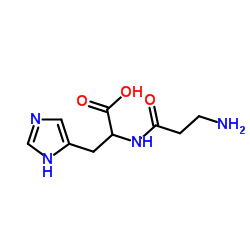 |
Phospholipase D
CAS:9001-87-0 |
|
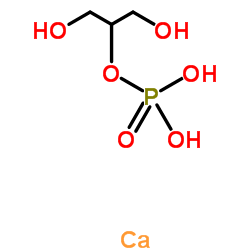 |
calcium 1,3-hydroxypropyl phosphate
CAS:58409-70-4 |