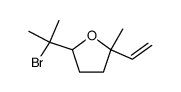
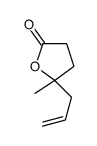
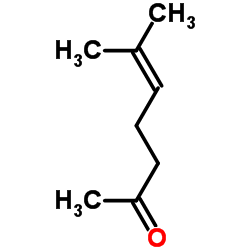
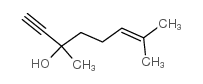
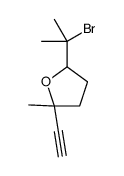
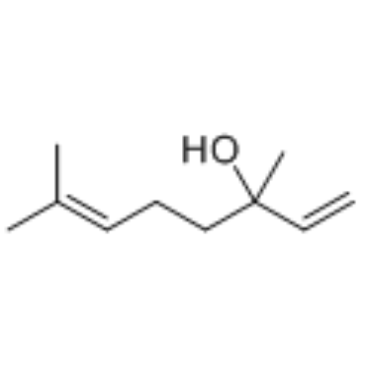
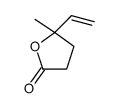
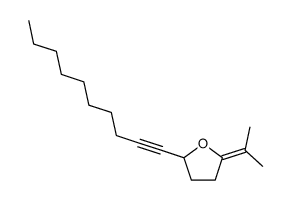
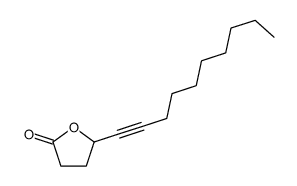
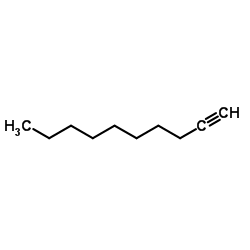
|
~78% |
|
~64% |
|
~% |
|
~93% |
|
~88% |
|
~% |
|
~% |
|
~65% |
|
~% |
|
~% |
|
~53% |
|
~% |
|
~% |
|
~% |
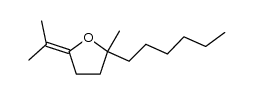
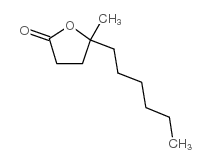

![2-(Bromomethyl)-1-oxaspiro[4.5]decane Structure](https://www.chemsrc.com/extcaspic/489/117038-86-5.png)