Stereoselective vinylogous Mannich reaction of 2-trimethylsilyloxyfuran with N-gulosyl nitrones.
Osamu Tamura, Kodai Takeda, Naka Mita, Masanori Sakamoto, Iwao Okamoto, Nobuyoshi Morita, Hiroyuki Ishibashi
Index: Org. Biomol. Chem. 9(21) , 7411-9, (2011)
Full Text: HTML
Abstract
Stereoselective vinylogous Mannich reaction of 2-trimethylsilyloxyfuran with L-gulose-derived chiral nitrones in the presence of a catalytic amount of trimethylsilyl trifluoromethanesulfonate was investigated. The selectivity was strongly influenced by the bulkiness of the C-substituent of the nitrone: for example, C-benzyloxymethyl nitrone afforded four stereoisomers, whereas bulky C-[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]nitrone gave a single stereoisomer. The latter product was elaborated to afford key synthetic intermediates for polyoxin C and dysiherbaine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
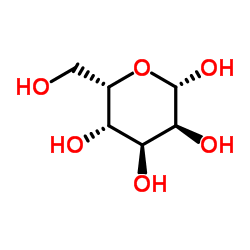 |
L-Gulose
CAS:6027-89-0 |
C6H12O6 |
|
Preparation of D-gulose from disaccharide lactitol using mic...
2013-01-01 [Biosci. Biotechnol. Biochem. 77(2) , 253-8, (2013)] |
|
The role of the gulose-mannose part of bleomycin in activati...
1988-07-15 [Biochem. J. 253(2) , 497-504, (1988)] |
|
Regio- and stereo-selective synthesis of carbohydrate isoxaz...
1992-03-16 [Carbohydr. Res. 226(1) , 49-56, (1992)] |
|
Microbial production of L-ascorbic acid from D-sorbitol, L-s...
2005-03-01 [Biosci. Biotechnol. Biochem. 69(3) , 659-62, (2005)] |
|
Gulose as a constituent of a glycoprotein.
1992-02-17 [FEBS Lett. 298(1) , 14-6, (1992)] |