The role of the gulose-mannose part of bleomycin in activation of iron-molecular oxygen complexes.
A Kénani, C Bailly, N Helbecque, J P Catteau, R Houssin, J L Bernier, J P Hénichart
Index: Biochem. J. 253(2) , 497-504, (1988)
Full Text: HTML
Abstract
A comparison of the complexing properties of metal ions and O2 activation by bleomycin-A2 (BLM-A2) and deglyco-BLM-A2 is presented. Deglyco-BLM-A2 is obtained from the parent derivative by HF cleavage of the sugar moiety followed by h.p.l.c. purification. Complexing of Cu(II) and Fe(III) is studied by using c.d. and e.s.r. spectroscopy. Spin-trapping experiments in the presence of phenyl N-t-butylnitrone indicated lower production of free radicals by deglyco-BLM-A2. Finally, a proposal is made to explain this discrepancy, focusing on the probable role of the gulose-mannose moiety acting as a protecting pocket, comparable with the pocket and picket-fence porphyrins described for haemoproteins.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
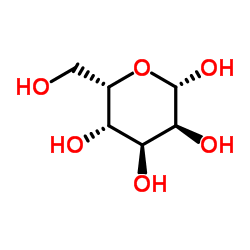 |
L-Gulose
CAS:6027-89-0 |
C6H12O6 |
|
Preparation of D-gulose from disaccharide lactitol using mic...
2013-01-01 [Biosci. Biotechnol. Biochem. 77(2) , 253-8, (2013)] |
|
Regio- and stereo-selective synthesis of carbohydrate isoxaz...
1992-03-16 [Carbohydr. Res. 226(1) , 49-56, (1992)] |
|
Microbial production of L-ascorbic acid from D-sorbitol, L-s...
2005-03-01 [Biosci. Biotechnol. Biochem. 69(3) , 659-62, (2005)] |
|
Stereoselective vinylogous Mannich reaction of 2-trimethylsi...
2011-11-07 [Org. Biomol. Chem. 9(21) , 7411-9, (2011)] |
|
Gulose as a constituent of a glycoprotein.
1992-02-17 [FEBS Lett. 298(1) , 14-6, (1992)] |