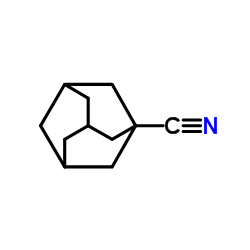
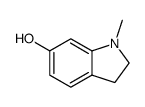
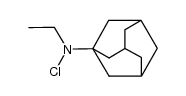
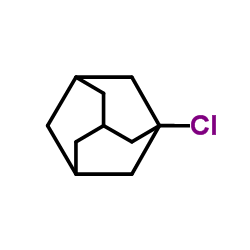
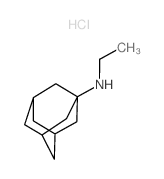
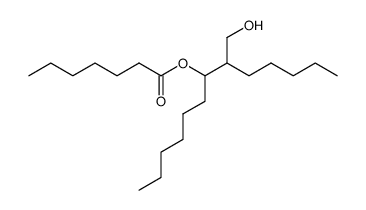
|
~90% |
|
~0% |
|
~97% |
|
~94% |
|
~57% |
|
~66% |
|
~30% |
|
~96% |
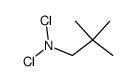
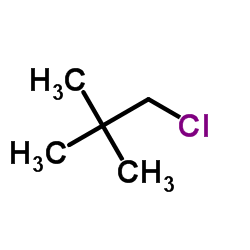
![N,N-Dichloro[(1-adamantyl)methyl]amine Structure](https://image.chemsrc.com/caspic/225/78685-91-3.png)