| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
4-(4-aminophenyl)aniline
CAS:92-87-5 |
|
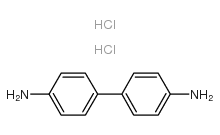 |
Benzidine dihydrochloride
CAS:531-85-1 |
|
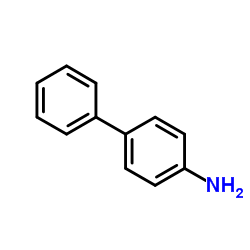 |
4-Aminobiphenyl
CAS:92-67-1 |