Baseline hepatitis B surface antigen quantitation can predict virologic response in entecavir-treated chronic hepatitis B patients.
Chia-Chi Wang, Tai-Chung Tseng, Pin-Chao Wang, Hans Hsienhong Lin, Jia-Horng Kao
Index: J. Formos. Med. Assoc. 113(11) , 786-93, (2014)
Full Text: HTML
Abstract
Several anti-viral drugs are approved for the treatment of hepatitis B virus (HBV) infection. However, whether quantitative hepatitis B surface antigen (qHBsAg) can predict the therapeutic response during long-term entecavir treatment remains unclear.Fifty-five chronic hepatitis B (CHB) patients who received entecavir for more than 2 years were enrolled. The serum qHBsAg level was measured by HBsAg II quant immunoassay. A significant decline in the qHBsAg level was defined as > 1 log reduction from baseline to 6 months of entecavir treatment.Of the 55 patients (41 males and 14 females with a mean age of 48.3 ± 11.4 years), 23 patients were positive for hepatitis B e antigen (HBeAg). The median treatment period was 34 months, and ranged from 26 months to 43 months. A total of 288 serum samples were used to determine the qHBsAg levels. At year 3 of entecavir therapy, one (1.8%) patient had HBsAg seroclearance. A high qHBsAg level was defined as greater than 10,000 IU/mL. Patients with a high baseline qHBsAg level had a lower rate of virologic response at year 1 (37.5% vs. 89.7%, p < 0.001) and year 2 (56.2% vs. 94.9%, p = 0.001). In this study population, 14.5% had a significant decline of the qHBsAg level. A significant decline could not predict HBeAg loss in HBeAg-positive or virologic response in all patients.The baseline serum qHBsAg level can predict virologic response in entecavir-treated CHB patients. However, a significant decline in the qHBsAg level cannot predict serologic or virologic response of entecavir treatment.Copyright © 2013. Published by Elsevier B.V.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
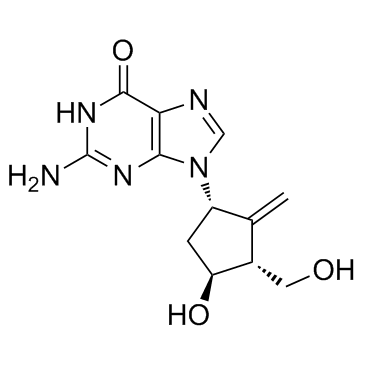 |
Entecavir
CAS:142217-69-4 |
C12H15N5O3 |
|
Formation of covalently closed circular DNA in Hep38.7-Tet c...
2014-09-26 [Biochem. Biophys. Res. Commun. 452(3) , 315-21, (2014)] |
|
Influence of the basal core promoter and precore mutation on...
2015-04-01 [J. Med. Virol. 87(4) , 601-8, (2015)] |
|
Decreased liver distribution of entecavir is related to down...
2015-04-25 [Eur. J. Pharm. Sci. 71 , 73-9, (2015)] |
|
Cost-effectiveness analysis of antiviral therapies for hepat...
2015-03-01 [Clin. Drug Investig. 35(3) , 197-209, (2015)] |
|
Hepatitis B Virus and DNA Stimulation Trigger a Rapid Innate...
2016-07-15 [J. Immunol. 197 , 630-43, (2016)] |