A quadruply hydrogen bonded heterocomplex displaying high-fidelity recognition.
Taiho Park, Eric M Todd, Shoji Nakashima, Steven C Zimmerman
Index: J. Am. Chem. Soc. 127(51) , 18133-42, (2005)
Full Text: HTML
Abstract
An exceptionally strong quadruply hydrogen-bonded complex is formed between 2,7-diamido-1,8-naphthyridine 3 (DAN) and the butylurea of guanosine 6 (UG) in chloroform. The UG unit can be prepared in four steps from guanosine on a 10 g scale in excellent yields without chromatographic purification. The association constant (Kassoc approximately 5 x 10(7) M(-1)) for the UG.DAN complex determined by fluorescence energy transfer from the naphthyridine unit of 3 to coumarin 343 covalently linked UG (18) is among the highest reported for a neutral DNA base-pair analogue. The weak self-association of DAN (Kdimer < 10 M(-1)) and UG (Kdimer ca. 200-300 M(-1)) means that the UG.DAN complex forms with unparalleled fidelity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
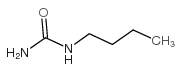 |
N-Butylurea
CAS:592-31-4 |
C5H12N2O |
|
Comparative evaluation of fifteen anti-sickling agents.
1983-04-01 [Blood 61(4) , 693-704, (1983)] |
|
Discovery of novel benzothiazolesulfonamides as potent inhib...
2003-11-03 [Bioorg. Med. Chem. 11(22) , 4769-77, (2003)] |
|
Inhibition of HIV-1 infection by alkylureas.
1991-12-01 [AIDS 5(12) , 1447-52, (1991)] |
|
Chalcogens as terminal ligands to iron: synthesis and struct...
2004-06-02 [J. Am. Chem. Soc. 126(21) , 6522-3, (2004)] |
|
Mechanistic studies on hydrotropic solubilization of nifedip...
1998-01-01 [Chem. Pharm. Bull. 46(1) , 125-30, (1998)] |