Site-specific metabolism of naphthalene and 1-nitronaphthalene in dissected airways of rhesus macaques.
Bridget Boland, Ching Yu Lin, Dexter Morin, Lisa Miller, Charles Plopper, Alan Buckpitt
Index: J. Pharmacol. Exp. Ther. 310(2) , 546-54, (2004)
Full Text: HTML
Abstract
Studies in rodents have demonstrated the importance of cytochrome P450 monooxygenases in generating reactive metabolites that produce Clara cell injury. Pulmonary P450 activities in rodents are much higher than those in primates, raising the issue of relevance of rodent data to primates. Few studies on P450-catalyzed activation of cytotoxicants in subcompartments of primate lung have been reported. Accordingly, infant monkey airway subcompartments, including trachea, proximal, midlevel, distal airways, and parenchyma, were incubated with naphthalene or 1-nitronaphthalene to define metabolism at both high (500 microM) and low (50 microM) substrate concentrations. There was a relatively even distribution of metabolizing activities for naphthalene across subcompartments, but at high concentrations of 1-nitronaphthalene, lower airways (midlevel airway through parenchyma) showed higher bioactivation than upper airways. Dihydrodiol was the predominant water-soluble metabolite of naphthalene generated by all subcompartments, whereas covalently bound metabolites accounted for the greatest percentage of 1-nitronaphthalene metabolites, especially in lower airways. As anticipated, the amounts of metabolite covalently bound as a percentage of total metabolite formed increased dramatically with the 10-fold increase in substrate concentration. With both substrates, the formation of water-soluble metabolites was approximately 100 times less than observed previously in rodents. We conclude that 1) there are significant quantitative differences between rhesus and rodents in substrate bioactivation; 2) the distribution of metabolizing activities for naphthalene but not 1-nitronaphthalene is significantly different for rodents and primates; and 3) a very high percentage of the metabolites generated, particularly for 1-nitronaphthalene, is bound covalently to cellular proteins.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
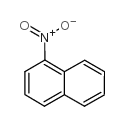 |
1-Nitronaphthalene
CAS:86-57-7 |
C10H7NO2 |
|
Combination of precolumn nitro-reduction and ultraperformanc...
2015-04-01 [J. Agric. Food Chem. 63(12) , 3161-7, (2015)] |
|
Determination of trace level genotoxic impurities in small m...
2014-09-26 [J. Chromatogr. A. 1361 , 217-28, (2014)] |
|
Nitropolycyclic aromatic hydrocarbons are inducers of mitoti...
2008-07-01 [Food Chem. Toxicol. 46(7) , 2344-8, (2008)] |
|
Selective detection of nitrated polycyclic aromatic hydrocar...
2000-01-01 [Rapid Commun. Mass Spectrom. 14(16) , 1474-81, (2000)] |
|
Induction of LacZ mutations in Muta Mouse can distinguish ca...
2006-09-19 [Mutat. Res. 608(1) , 88-96, (2006)] |