Metabolism of alcohol and ketone by cytochrome P-450 oxygenase: fluoren-9-ol in equilibrium with fluoren-9-one.
C Chen, R C Lefers, E L Brough, D P Gurka
Index: Drug Metab. Dispos. 12(4) , 421-6, (1984)
Full Text: HTML
Abstract
Fluoren-9-ol and fluoren-9-one were used as model substrates to study microsomal metabolism of alcohols and carbonyl compounds. It was found that there was an oxidoreductase(s) present in the microsomal preparation that catalyzed interconversion of this alcohol and ketone using pyridine nucleotides as cofactors. The alcohol could also be oxygenated to the ketone by microsomes and NADPH. It required molecular oxygen. This oxygenation was inhibited by SKF 525-A and CO, suggesting the involvement of P-450 oxygenase. Using highly purified P-450 oxygenase components, it was shown that fluoren-9-ol was oxygenated by the complete system to fluoren-9-one. Exclusion of cytochrome P-450 or its reductase resulted in no oxygenation. Dilauroylglyceryl-3-phosphorylcholine was without effect on the reaction. Fluoren-9-one, on the other hand, was reduced to fluoren-9-ol by NADPH-cytochrome P-450 reductase and NADPH. It is suggested that other alcohols and carbonyl compounds are similarly metabolized.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
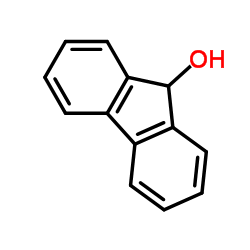 |
9-fluorenol
CAS:1689-64-1 |
C13H10O |
|
Rational design of indoleamine 2,3-dioxygenase inhibitors.
2010-02-11 [J. Med. Chem. 53 , 1172-89, (2010)] |
|
A gold nanoparticle-based colorimetric probe for rapid detec...
2015-07-07 [Analyst 140 , 4662-7, (2015)] |
|
Assessing hazardous risks of indoor airborne polycyclic arom...
2016-01-15 [Clin. Chim. Acta 452 , 204-13, (2015)] |
|
A transcriptional luxAB reporter fusion responding to fluore...
2001-12-01 [Res. Microbiol. 152(10) , 849-59, (2001)] |
|
Wake promoting agents: Search for next generation modafinil,...
2012-06-01 [Bioorg. Med. Chem. Lett. 22(11) , 3751-3, (2012)] |