Activation of hydrazine derivatives to free radicals in the perfused rat liver: a spin-trapping study.
B K Sinha
Index: Biochim. Biophys. Acta 924(2) , 261-9, (1987)
Full Text: HTML
Abstract
Spin-trapping techniques and electron spin resonance spectroscopy have been used to study bioactivations of hydrazine and its derivatives by isolated perfused rat livers. Using phenylbutylnitrone (PBN) as the stable spin trap, it was found that the liver perfusion of hydrazine, acetylhydrazine and isoniazid resulted in the formation of the same carbon-centered radical which was shown to be the acetyl radical. The identity of the acetyl radical was confirmed after organic extraction of the liver perfusates, by comparing its coupling constants with those of the in vitro metal ion- or horseradish peroxidase-catalyzed oxidation products of the hydrazines in the same solvents. The liver perfusion of iproniazid formed the isopropyl radical which was previously observed to result from peroxidase-catalyzed oxidation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
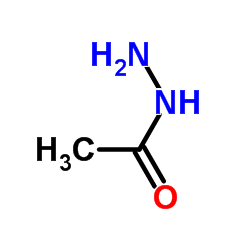 |
Acethydrazide
CAS:1068-57-1 |
C2H6N2O |
|
Stable isotopic labeling-based quantitative targeted glycomi...
2015-01-01 [Biotechnol. Prog. 31 , 840-8, (2015)] |
|
Development and characterization of Haemophilus influenzae t...
2015-05-28 [Vaccine 33 , 2646-54, (2015)] |
|
Quantitative glycome analysis of N-glycan patterns in bladde...
2015-02-06 [J. Proteome Res. 14(2) , 639-53, (2015)] |
|
Human PXR modulates hepatotoxicity associated with rifampici...
2013-04-01 [Nat. Med. 19(4) , 418-20, (2013)] |
|
Pharmacokinetics of the toxic hydrazino metabolites formed f...
1985-12-01 [J. Pharmacol. Exp. Ther. 235(3) , 566-70, (1985)] |