| Structure | Name/CAS No. | Articles |
|---|---|---|
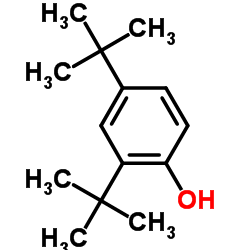 |
2,4-Di-t-butylphenol
CAS:96-76-4 |
|
 |
3,5-Bis-tert-butylsalicylic acid
CAS:19715-19-6 |