| Structure | Name/CAS No. | Articles |
|---|---|---|
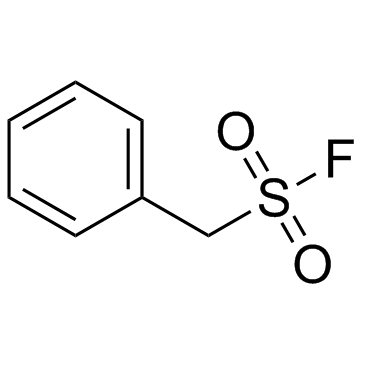 |
PMSF
CAS:329-98-6 |
|
 |
Glycerol
CAS:56-81-5 |
|
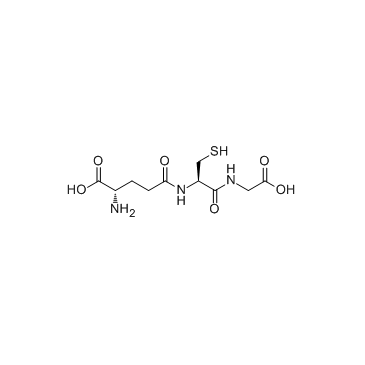 |
Glutathione
CAS:70-18-8 |
|
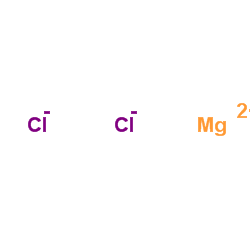 |
Magnesium choride
CAS:7786-30-3 |
|
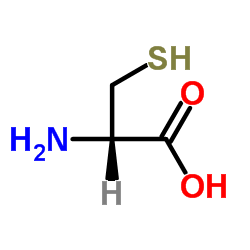 |
D-Cysteine
CAS:921-01-7 |
|
 |
Maltose
CAS:69-79-4 |