Novel triiodophenol derivatives induce caspase-independent mitochondrial cell death in leukemia cells inhibited by Myc.
Matilde Parreño, Jose P Vaqué, Isolda Casanova, Pilar Frade, M Virtudes Céspedes, Miguel Angel Pavón, Antoni Molins, Mercedes Camacho, Luis Vila, Josep F Nomdedeu, Ramon Mangues, Javier León
Index: Mol. Cancer Ther. 5(5) , 1166-75, (2006)
Full Text: HTML
Abstract
2,4,6-Triiodophenol (Bobel-24, AM-24) was originally described as a nonsteroid antiinflammatory molecule. We have synthesized three derivatives of Bobel-24 (Bobel-4, Bobel-16, and Bobel-30) and tested their activities as putative antileukemic agents. We have found that Bobel-24 and Bobel-16 were dual inhibitors of cyclooxygenase and 5-lipoxygenase, whereas Bobel-4 and Bobel-30 were selective against 5-lipoxygenase. We have tested the antiproliferative activity of these compounds on a panel of cell lines derived from myeloid and lymphoid leukemias (K562, Raji, HL-60, and Molt4). The cytotoxic IC(50) in these cell lines ranged between 14 and 50 micromol/L, but it was higher for nontransformed cells such as 32D, NIH3T3, or human leukocytes. All compounds showed cytotoxic activity on all tested cell lines, accompanied by DNA synthesis inhibition and arrest in the G(0)/G(1) phase. Bobel-16, Bobel-4, and Bobel-24 induced a caspase-independent cell death in K562 and Raji cells, accompanied by chromatin condensation, cytochrome c release, and dissipation of mitochondrial membrane potential in a concentration-dependent manner and production of reactive oxygen species. As the proto-oncogene MYC is involved in mitochondrial biogenesis and survival of leukemia cells, we tested its effect on bobel activity. Bobel-24 induced down-regulation of MYC in K562 and, consistently, ectopic expression of MYC results in partial protection towards the cytotoxic effect of Bobel-24. In conclusion, Bobel derivatives induce a caspase- and Bcl-2-independent cell death in which mitochondrial permeabilization and MYC down-regulation are involved. Bobels may serve as prototypes for the development of new agents for the therapy of leukemia.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
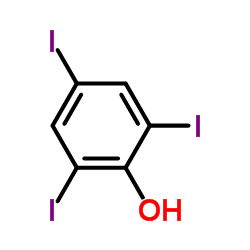 |
2,4,6-Triiodophenol
CAS:609-23-4 |
C6H3I3O |
|
Distribution of dehalogenation activity in subseafloor sedim...
2013-04-19 [Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368(1616) , 20120249, (2013)] |
|
Comparative developmental toxicity of new aromatic halogenat...
2013-10-01 [Environ. Sci. Technol. 47(19) , 10868-76, (2013)] |
|
Fast speciation analysis of iodophenol compounds in river wa...
2004-06-01 [Electrophoresis 25(12) , 1843-51, (2004)] |
|
Halogenated phenolic contaminants inhibit the in vitro activ...
2011-12-01 [Toxicol. Sci. 124(2) , 339-47, (2011)] |
|
Characterization of plasma triiodophenol binding proteins in...
2011-04-01 [Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 153(3) , 328-35, (2011)] |