Dealing with time-dependent pharmacokinetics during the early clinical development of a new leukotriene B4 synthesis inhibitor.
Iñaki F Trocóniz, Ilonka Zsolt, María J Garrido, Marta Valle, Rosa M Antonijoan, Manel J Barbanoj
Index: Pharm. Res. 23(7) , 1533-42, (2006)
Full Text: HTML
Abstract
The aim of this study was to explore the possibility of achieving a practical dosing regimen for 2,4,6-triiodophenol (AM-24), a new leukotriene B4 (LTB4) synthesis inhibitor. First, a model capable of dealing with the nonlinearity in its pharmacokinetic profile was built, and then it was combined with a pharmacodynamic model previously established with data from earlier phase I trials.One week after the first 240-, 350-, or 500-mg oral dose of AM-24, six additional doses were given to 24 healthy volunteers once daily. A total of 33 blood samples were obtained from each individual. Different models, including enzyme turnover models, were fitted to the data by using the software NONMEM.Drug absorption was modeled with a first-order process. Drug disposition was described with a one-compartment model, and elimination with an (auto)inhibited and a noninhibited clearance. AM-24 inhibited the enzyme production rate to a maximum of 98%. Relative bioavailability was independent of the decrease in the amount of enzyme. The estimate of the enzyme turnover half-life was 8.5 h.Simulations have shown that steady-state conditions eliciting 90% of maximal LTB4 synthesis inhibition can be reached after 3 weeks during an oral treatment with AM-24 administered at the dosage of 500 mg once daily.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
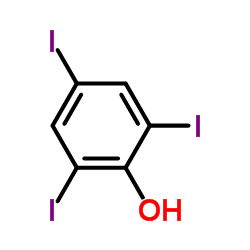 |
2,4,6-Triiodophenol
CAS:609-23-4 |
C6H3I3O |
|
Distribution of dehalogenation activity in subseafloor sedim...
2013-04-19 [Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368(1616) , 20120249, (2013)] |
|
Comparative developmental toxicity of new aromatic halogenat...
2013-10-01 [Environ. Sci. Technol. 47(19) , 10868-76, (2013)] |
|
Fast speciation analysis of iodophenol compounds in river wa...
2004-06-01 [Electrophoresis 25(12) , 1843-51, (2004)] |
|
Halogenated phenolic contaminants inhibit the in vitro activ...
2011-12-01 [Toxicol. Sci. 124(2) , 339-47, (2011)] |
|
Characterization of plasma triiodophenol binding proteins in...
2011-04-01 [Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 153(3) , 328-35, (2011)] |