| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Resveratrol
CAS:501-36-0 |
|
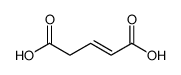 |
GLUTACONIC ACID
CAS:628-48-8 |
|
 |
trans-Glutaconic acid
CAS:1724-02-3 |