| Structure | Name/CAS No. | Articles |
|---|---|---|
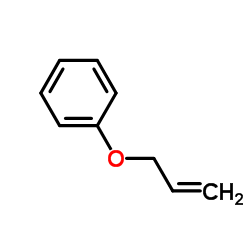 |
(Allyloxy)benzene
CAS:1746-13-0 |
|
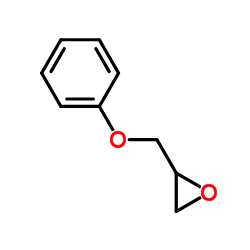 |
phenyl glycidyl ether
CAS:122-60-1 |