| Structure | Name/CAS No. | Articles |
|---|---|---|
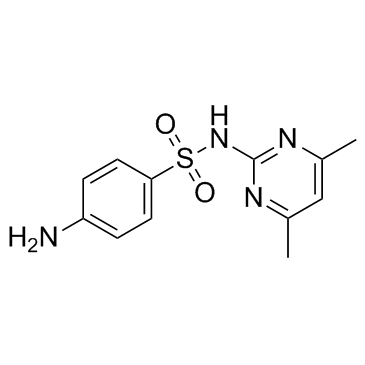 |
Sulfamethazine
CAS:57-68-1 |
|
 |
(S)-(+)-Epichlorhydrin
CAS:67843-74-7 |
|
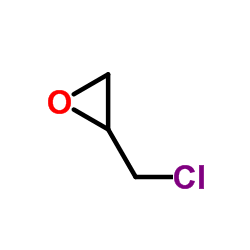 |
Epichlorohydrin
CAS:106-89-8 |
|
 |
Sartorius SM 11127
CAS:9004-35-7 |