| Structure | Name/CAS No. | Articles |
|---|---|---|
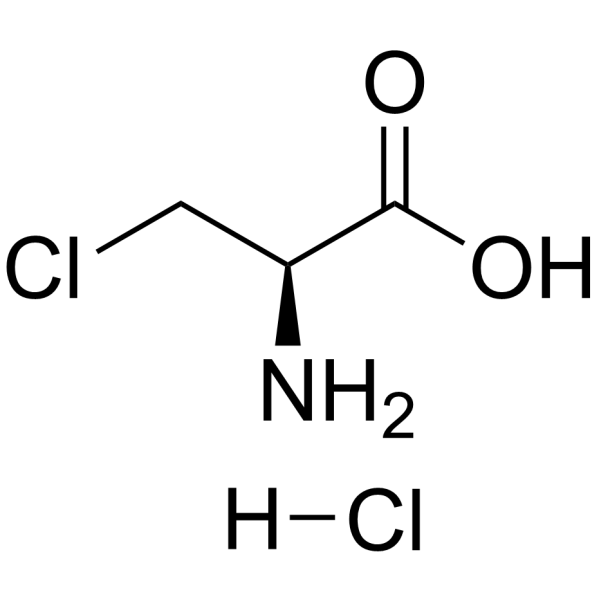 |
3-Chloro-L-Alanine Hydrochloride
CAS:51887-89-9 |
|
 |
(S)-2-Amino-3-chloropropanoic acid hydrochloride
CAS:51887-88-8 |