| Structure | Name/CAS No. | Articles |
|---|---|---|
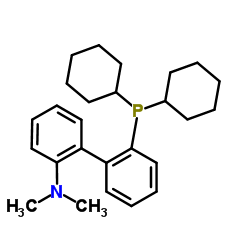 |
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl
CAS:213697-53-1 |
|
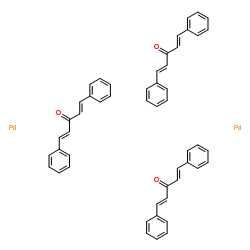 |
Tris(dibenzylideneacetone)dipalladium(0)
CAS:51364-51-3 |
|
 |
2-Bromoacetamide
CAS:683-57-8 |