Natural Product Research
2013-01-01
An efficient total synthesis of ruprechstyril from Ruprechtia tangarana.
Aamer Saeed
Index: Nat. Prod. Res. 27(13) , 1153-8, (2013)
Full Text: HTML
Abstract
A short total synthesis of natural isocarbostyril ruprechstyril (3-n-pentyl-6-methoxy-8-hydroxy-1(2H)-isoquinolinone) isolated from Ruprechtia tangarana is reported. 6,8-Dimethoxy-3-pentylisocoumarin obtained by condensation of 3,5-dimethoxyhomophthalic anhydride with hexanoyl chloride was smoothly converted to O-methylruprechstyril by refluxing with methanamide. Regioselective demethylation of the latter using anhydrous aluminium chloride in dichloromethane furnished the ruprechstyril. Complete demethylation to give (6-desmethoxyruprechstyril) was achieved using same reagent in ethanethiol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
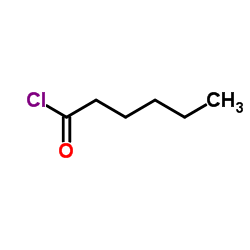 |
Hexanoyl chloride TOP1 supplier
CAS:142-61-0 |
C6H11ClO |
Related Articles:
More...
|
Remarkably regioselective deacylation of cellulose esters us...
2014-10-13 [Carbohydr. Polym. 111 , 25-32, (2014)] |
|
Use of multivariate statistical techniques to optimize the s...
2015-03-01 [Talanta 134 , 256-63, (2015)] |
|
One-step dispersion of cellulose nanofibers by mechanochemic...
2012-12-01 [ChemSusChem 5(12) , 2319-22, (2012)] |
|
A facile asymmetric synthesis of (s)-14-methyl-1-octadecene,...
2013-01-01 [Molecules 18(5) , 5201-8, (2013)] |
|
Total synthesis and antibacterial screening of ( ± )-7-butyl...
2013-01-01 [J. Asian Nat. Prod. Res. 15(10) , 1112-22, (2013)] |