| Structure | Name/CAS No. | Articles |
|---|---|---|
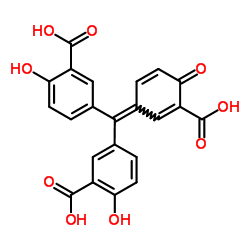 |
Aurintricarboxylic acid
CAS:4431-00-9 |
|
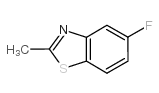 |
5-Fluoro-2-methylbenzothiazole
CAS:399-75-7 |