Journal of Organic Chemistry
2010-05-21
A one-step synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid esters from o-nitrobenzaldehydes.
Hariharan Venkatesan, Frances M Hocutt, Todd K Jones, Michael H Rabinowitz
Index: J. Org. Chem. 75(10) , 3488-91, (2010)
Full Text: HTML
Abstract
A straightforward and efficient one-step procedure for the synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid ethyl esters is described. The simple reductive cyclization is carried out by treating various substituted o-nitrobenzaldehydes with inexpensive, commercially available 3,3-diethoxypropionic acid ethyl ester and SnCl(2).2H(2)O in refluxing ethanol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
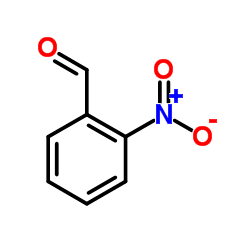 |
2-Nitrobenzaldehyde
CAS:552-89-6 |
C7H5NO3 |
Related Articles:
More...
|
A selective biomarker for confirming nitrofurazone residues ...
2015-12-01 [Anal. Bioanal. Chem 407 , 8971-7, (2015)] |
|
Determination of nitrofuran and chloramphenicol residues by ...
2015-03-03 [Anal. Chim. Acta 862 , 41-52, (2015)] |
|
Tenellin acts as an iron chelator to prevent iron-generated ...
2015-01-01 [FEMS Microbiol. Lett. 362 , 1-8, (2015)] |
|
Generation of semicarbazide from natural azine development i...
2015-01-01 [Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 32 , 1416-30, (2015)] |
|
Fortified versus incurred residues: extraction of furazolidi...
2015-06-01 [Anal. Bioanal. Chem 407 , 4535-40, (2015)] |