| Structure | Name/CAS No. | Articles |
|---|---|---|
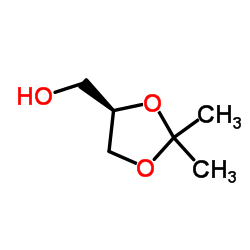 |
(R)-(-)-Solketal
CAS:14347-78-5 |
|
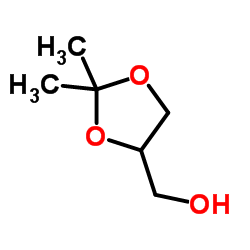 |
Solketal
CAS:100-79-8 |
|
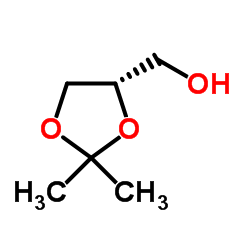 |
(S)-(+)-Solketal
CAS:22323-82-6 |