| Structure | Name/CAS No. | Articles |
|---|---|---|
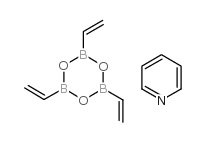 |
Vinylboronic anhydride pyridine complex
CAS:442850-89-7 |
|
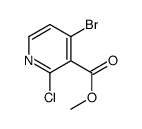 |
Methyl 4-bromo-2-chloronicotinate
CAS:1064678-14-3 |