Reinvestigation of the reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone.
A Ravagli, G Boschin, L Scaglioni, A Arnoldi
Index: J. Agric. Food Chem. 47(12) , 4962-9, (1999)
Full Text: HTML
Abstract
The reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone was reinvestigated as a part of a systematic study on low molecular weight colored compounds from the Maillard reaction. In acetic acid/piperidine, besides 2-(2-furanylmethylene)-4-hydroxy-5-methyl-3(2H)-furanone (1) and 5-[2-(2-furanyl)ethenyl]-2-(2-furanylmethylene)-4-hydroxy-5-methyl -3( 2H)-furanone (2), four novel compounds, 15a, 15b, 16a, and 16b, were isolated and characterized. These compounds are produced from two molecules of furanone 1 and one molecule of 2-furancarboxaldehyde, and a mechanism is proposed for their formation. Compounds 1, 15a, 15b, 16a, and 16b are formed also by reacting 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone in water at pH 3 and 2, whereas 2 was never detected. The formation of these compounds was studied also in xylose/lysine and xylose/glycine model systems.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
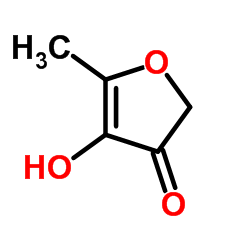 |
4-hyroxy-5-methyl-3-furanone
CAS:19322-27-1 |
C5H6O3 |
|
Norfuraneol dephosphorylates eNOS at threonine 495 and enhan...
2009-03-01 [Cardiovasc. Res. 81(4) , 750-7, (2009)] |
|
Investigation of the reaction between 4-hydroxy-5-methyl-3(2...
1999-04-01 [J. Agric. Food Chem. 47(4) , 1626-34, (1999)] |
|
Heterocyclic volatiles formed by heating cysteine or hydroge...
2001-02-01 [J. Agric. Food Chem. 49(2) , 816-22, (2001)] |
|
Maillard reaction products modulating the growth of human tu...
2003-01-01 [Chem. Res. Toxicol. 16(1) , 48-55, (2003)] |
|
LuxS: its role in central metabolism and the in vitro synthe...
2002-04-01 [Microbiology 148(Pt 4) , 909-22, (2002)] |