Heterocyclic volatiles formed by heating cysteine or hydrogen sulfide with 4-hydroxy-5-methyl-3(2H)-furanone at pH 6.5.
F B Whitfield, D S Mottram
Index: J. Agric. Food Chem. 49(2) , 816-22, (2001)
Full Text: HTML
Abstract
The reaction of 4-hydroxy-5-methyl-3(2H)-furanone (HMF) with cysteine or hydrogen sulfide at pH 6.5 for 60 min at 140 degrees C produced complex mixtures of volatile compounds, the majority of these containing either sulfur or nitrogen. Of the 68 compounds detected, 63 were identified, some tentatively, by GC-MS. Among the identified compounds were thiophenes (10), thiophenones (6), thienothiophenes (5), thiazoles (5), trithiolanes (4), pyrazines (6), and oxazoles (4). More compounds were produced in the reaction of HMF with cysteine (63) than were formed in the reaction with hydrogen sulfide (33). In both systems, thiophenones were major reaction products, accounting for 25-36% of the total volatiles formed. Possible reasons for the differences in the composition of the two systems are discussed. The contributions of these reactions, and their products, to the flavor of heated foods are considered.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
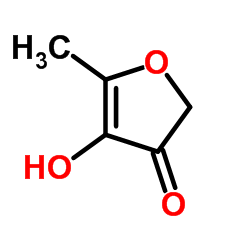 |
4-hyroxy-5-methyl-3-furanone
CAS:19322-27-1 |
C5H6O3 |
|
Norfuraneol dephosphorylates eNOS at threonine 495 and enhan...
2009-03-01 [Cardiovasc. Res. 81(4) , 750-7, (2009)] |
|
Investigation of the reaction between 4-hydroxy-5-methyl-3(2...
1999-04-01 [J. Agric. Food Chem. 47(4) , 1626-34, (1999)] |
|
Maillard reaction products modulating the growth of human tu...
2003-01-01 [Chem. Res. Toxicol. 16(1) , 48-55, (2003)] |
|
LuxS: its role in central metabolism and the in vitro synthe...
2002-04-01 [Microbiology 148(Pt 4) , 909-22, (2002)] |
|
An efficient flow-photochemical synthesis of 5H-furanones le...
2012-04-27 [Angew. Chem. Int. Ed. Engl. 51(18) , 4405-8, (2012)] |