| Structure | Name/CAS No. | Articles |
|---|---|---|
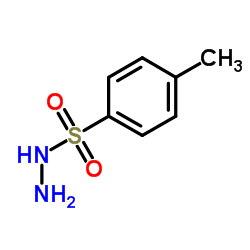 |
4-Methylbenzenesulfonhydrazide
CAS:1576-35-8 |
Anna V Gudmundsdottir, Mark Nitz
Index: Org. Lett. 10(16) , 3461-3, (2008)
Full Text: HTML
N'-Glycopyranosylsulfonohydrazides are introduced as glycosyl donors for protecting group free synthesis of O-glycosides, glycosyl azides, and oxazolines. Mono- and disaccharides containing a reducing terminal N-acetylglucosamine residue were condensed with p-toluenesulfonylhydrazide to give the desired beta- d-pyranose donors. These donors can be activated with NBS and then glycosidated with the desired alcohol or transformed to the oxazoline or glycosyl azide.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Methylbenzenesulfonhydrazide
CAS:1576-35-8 |
C7H10N2O2S |
|
Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and h...
2010-11-01 [Eur. J. Med. Chem. 45(11) , 5080-5, (2010)] |
|
Vasoconstrictor-induced changes in renal blood flow: role of...
1988-04-01 [Am. J. Physiol. 254(4 Pt 2) , F470-6, (1988)] |
|
Mutagenic activity of p-toluenesulfonhydrazide.
1992-01-01 [Toxicol. Ind. Health 8(6) , 369-76, (1992)] |
|
Enantioselective synthesis of trifluoromethyl-substituted cy...
2007-07-05 [Org. Lett. 9 , 2625, (2007)] |
|
Dipyrazolo[1,5-a:4',3'-c]pyridines - a new heterocyclic syst...
2012-01-01 [Beilstein J. Org. Chem. 8 , 2223-9, (2012)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved