| Structure | Name/CAS No. | Articles |
|---|---|---|
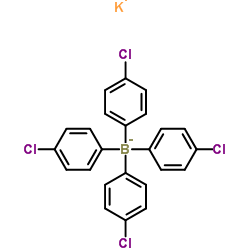 |
Potassium tetrakis(4-chlorophenyl)borate(1-)
CAS:14680-77-4 |
|
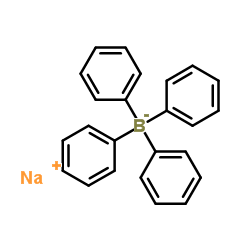 |
Sodium tetraphenylborate
CAS:143-66-8 |