Engineering anthracycline biosynthesis toward angucyclines.
Mikko Metsä-Ketelä, Kaisa Palmu, Tero Kunnari, Kristiina Ylihonko, Pekka Mäntsälä
Index: Antimicrob. Agents Chemother. 47(4) , 1291-6, (2003)
Full Text: HTML
Abstract
The biosynthesis pathways of two anthracyclines, nogalamycin and aclacinomycin, were directed toward angucyclines by using an angucycline-specific cyclase, pgaF, isolated from a silent antibiotic biosynthesis gene cluster. Addition of pgaF to a gene cassette that harbored the early biosynthesis genes of nogalamycin resulted in the production of two known angucyclinone metabolites, rabelomycin and its precursor, UWM6. Substrate flexibility of pgaF was demonstrated by replacement of the nogalamycin minimal polyketide synthase genes in the gene cassette with the equivalent aclacinomycin genes together with aknE2 and aknF, which specify the unusual propionate starter unit in aclacinomycin biosynthesis. This modification led to the production of a novel angucyclinone, MM2002, in which the expected ethyl side chain was incorporated into the fourth ring.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
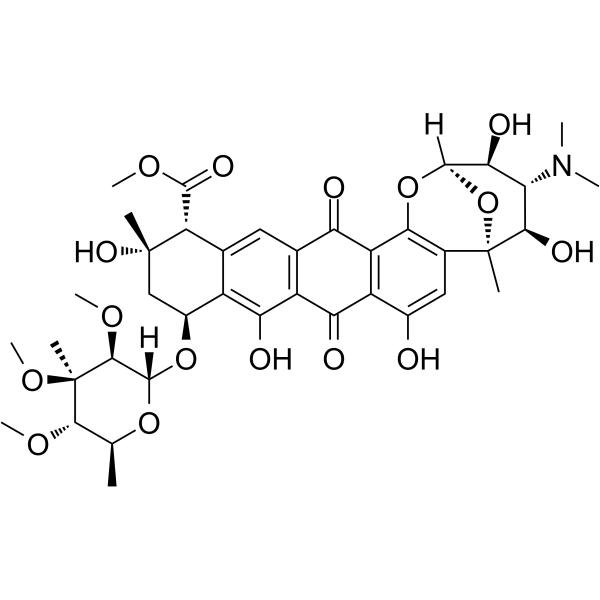 |
Nogalamycin
CAS:1404-15-5 |
C39H49NO16 |
|
Kinetic characterization of small DNA-binding molecules inte...
2016-01-01 [Anal. Biochem. 492 , 34-42, (2015)] |
|
The intercalation of DNA double helices with doxorubicin and...
2007-07-01 [J. Mol. Graph. Model. 26(1) , 14-9, (2007)] |
|
Expression, purification and crystallization of the cofactor...
2009-03-01 [Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65(Pt 3) , 256-9, (2009)] |
|
Influence of initial charge state on fragmentation patterns ...
2005-10-01 [J. Mass Spectrom. 40(10) , 1362-71, (2005)] |
|
Discovery of a Two-Component Monooxygenase SnoaW/SnoaL2 Invo...
2012-05-25 [Chem. Biol. 19(5) , 638-46, (2012)] |