The intercalation of DNA double helices with doxorubicin and nogalamycin.
Vernon G S Box
Index: J. Mol. Graph. Model. 26(1) , 14-9, (2007)
Full Text: HTML
Abstract
A variety of molecules bind to DNA in its major and minor grooves, and some, like the anthraquinoids, are known to form intercalates in which these molecules are inserted directly into the double helix, between the bases. Several researchers have pointed to an electron transfer mechanism (leading to ion pairing) as one of the factors that could hold the intercalated entities like doxorubicin in place, but the bulky anthraquinone nogalamycin did not seem to become engaged in electron transfer. The molecular modeling program STR3DI32 was used to investigate the stabilities of these intercalated anthraquinone before any possible electron transfer has occurred.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
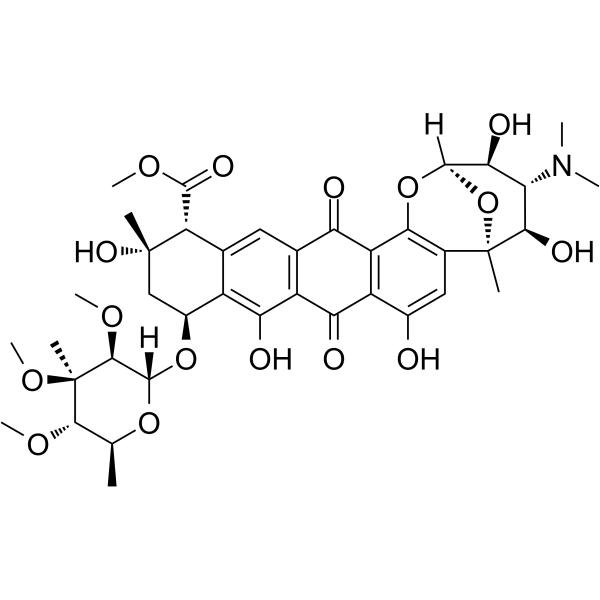 |
Nogalamycin
CAS:1404-15-5 |
C39H49NO16 |
|
Kinetic characterization of small DNA-binding molecules inte...
2016-01-01 [Anal. Biochem. 492 , 34-42, (2015)] |
|
Engineering anthracycline biosynthesis toward angucyclines.
2003-04-01 [Antimicrob. Agents Chemother. 47(4) , 1291-6, (2003)] |
|
Expression, purification and crystallization of the cofactor...
2009-03-01 [Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65(Pt 3) , 256-9, (2009)] |
|
Influence of initial charge state on fragmentation patterns ...
2005-10-01 [J. Mass Spectrom. 40(10) , 1362-71, (2005)] |
|
Discovery of a Two-Component Monooxygenase SnoaW/SnoaL2 Invo...
2012-05-25 [Chem. Biol. 19(5) , 638-46, (2012)] |