Enhancement of enzyme activity and enantioselectivity via cultivation in nitrile metabolism by Rhodococcus sp. CGMCC 0497.
Zhong-Liu Wu, Zu-Yi Li
Index: Biotechnol. Appl. Biochem. 35(Pt 1) , 61-7, (2002)
Full Text: HTML
Abstract
Racemic 2-phenylpropionitrile was resolved enantioselectively by nitrile-converting enzymes in cells of Rhodococcus sp. CGMCC 0497 to S-(+)-2-phenylpropionic acid and R-(-)-2-phenylpropionamide. By optimization of the culture conditions, great enhancement of enzyme activity and enantioselectivity was achieved. Furthermore, the relationship between cell-growth periodicity and enzyme accumulation was studied; the addition of inducer was delayed by 1 day and the reaction was further improved. This unusual strategy has almost never been reported with other nitrile-converting strains. The resulting culture broth, containing methacrylamide as the inducer, beef extract as the nitrogen source and glucose as the carbon source, with methacrylamide added 24 h later, seemed to be most suitable. S-(+)-2-Phenylpropionic acid and R-(-)-2-phenylpropionamide were produced with yields of 48% (enantiomeric excess, 96%) and 42% (enantiomeric excess, 97%) respectively with no nitrile left after 3 h, or with yields of 52% and 39% (enantiomeric excess, 93% and 99%) respectively after 6 h.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
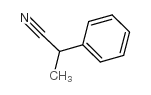 |
ALPHA-METHYLPHENYLACETONITRILE
CAS:1823-91-2 |
C9H9N |
|
Conversion of sterically demanding α,α-disubstituted phenyla...
2012-01-01 [Appl. Environ. Microbiol. 78 , 48 - 57, (2012)] |
|
Heterologous expression, purification and characterization o...
2011-01-01 [BMC Biotechnol. 11 , 2, (2011)] |
|
Production of S-(+)-2-phenylpropionic acid from (R,S)-2-phen...
1993-08-01 [Appl. Microbiol. Biotechnol. 39(6) , 720-5, (1993)] |
|
Utilization of arylaliphatic nitriles by haloalkaliphilic Ha...
2008-11-01 [Appl. Microbiol. Biotechnol. 81(2) , 371-8, (2008)] |