| Structure | Name/CAS No. | Articles |
|---|---|---|
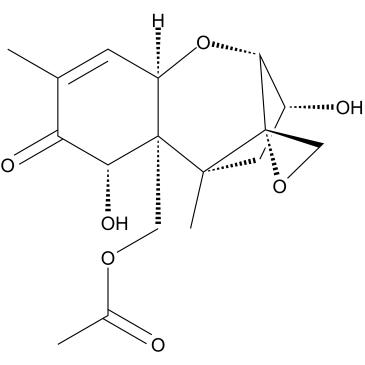 |
15-Acetyl-deoxynivalenol
CAS:88337-96-6 |
|
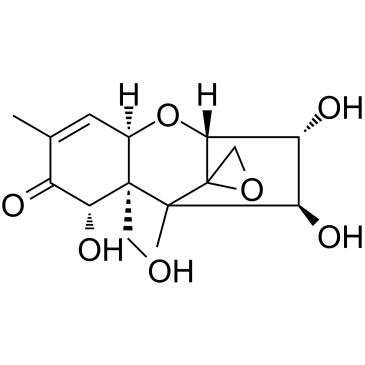 |
Nivalenol
CAS:23282-20-4 |
|
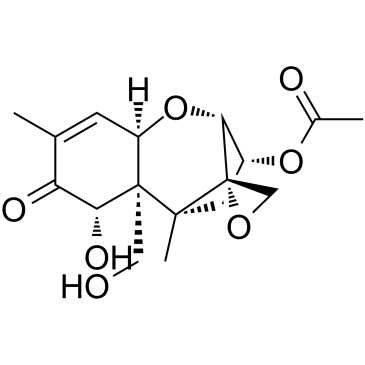 |
Trichothec-9-en-8-one,3-(acetyloxy)-12,13-epoxy-7,15-dihydroxy-, (3a,7a)
CAS:50722-38-8 |
|
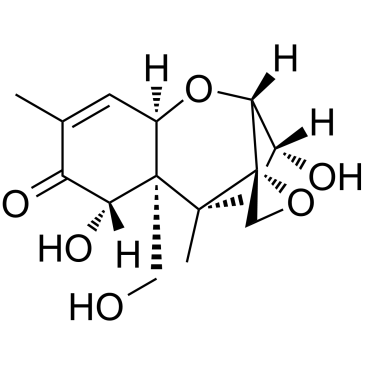 |
DEOXYNIVALENOL
CAS:51481-10-8 |