Enhanced solubilization of arsenic and 2,3,4,6 tetrachlorophenol from soils by a cyclodextrin derivative.
V Chatain, K Hanna, C de Brauer, R Bayard, P Germain, V. Chatain, K. Hanna, C. de Brauer, R. Bayard, P. Germain
Index: Chemosphere 57(3) , 197-206, (2004)
Full Text: HTML
Abstract
The application of extracting aqueous solutions with cyclodextrins in several soil remediation technologies has been increasingly studied but little is known about their removal capacities toward the inorganic species. Herein, the effectiveness of cyclodextrins (CDs) in extracting arsenic, copper, and iron from a mining soil is presented. In a preliminary test of four types of CD aqueous solutions, only the addition of carboxylmethyl-beta-cyclodextrin CMCD (a cyclodextrin derivative) led to a significant enhancement in arsenic removal. An increase in the concentration of copper and iron in the leachates was also observed with CMCD. Kinetic study of arsenic release was carried out at two temperatures (20 and 35 degrees C). The arsenic concentration in the leachates increases with increasing cyclodextrin concentration. At an 80 mM CMCD concentration, arsenic, copper, and iron released in filtrates were about 20-, 1,000-, and 4,000-fold greater, respectively, than that obtained using deionized water. In the soil system, the CMCD capacity removal was found to be higher for cations than for arsenic. Because the tetrachlorophenol can co-occur with arsenic and copper in several contaminated sites, its solubilization by CMCD was also investigated. Extraction experiments were performed to extract 2,3,4,6 tetrachlorophenol (TeCP) in spiked soil with CMCD. The results of batch experiments have shown that CMCD could significantly increase the TeCP extraction from soil. CD sorption on soils as quantified by a fluorescence technique was low, indicating no significant loss of CD during the leaching experiments. The use of CMCD as a flushing agent to enhance the removal of both inorganic and organic pollutants from mixed-contaminated soils appears as a promising remediation method.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
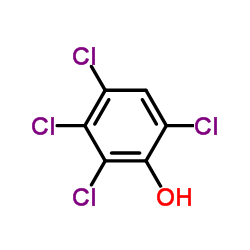 |
2,3,4,6-TETRACHLOROPHENOL
CAS:58-90-2 |
C6H2Cl4O |
|
PCDD/F formation from chlorophenols by lignin and manganese ...
2014-09-01 [Chemosphere 110 , 129-35, (2014)] |
|
Comparison of stir bar sorptive extraction and solid-phase m...
2008-05-15 [Talanta 75(3) , 753-9, (2008)] |
|
In situ polychlorophenol bioremediation potential of the ind...
2001-07-01 [Water Res. 35(10) , 2496-504, (2001)] |
|
Chemical kinetic modelling of PCDD formation from chlorophen...
2000-09-01 [Chemosphere 41(6) , 943-51, (2000)] |
|
Metabolism of Halophenols by 2,4,5-trichlorophenoxyacetic ac...
1983-11-01 [Appl. Environ. Microbiol. 46(5) , 1176-81, (1983)] |