Chemical kinetic modelling of PCDD formation from chlorophenol catalysed by incinerator fly ash.
H Huang, A Buekens
Index: Chemosphere 41(6) , 943-51, (2000)
Full Text: HTML
Abstract
A kinetic model is developed for PCDD formation from chlorophenol catalysed by incinerator fly ash. The key step in the model is a Langmuir-Hinshelwood type elementary step for the coupling of two adsorbed chlorophenol species to PCDD. Kinetic expression is derived which can relate PCDD formation rates with process variables including temperature, precursor concentration, fly ash loading and number of active sites in fly ash. Calculated PCDD formation rates based on this kinetic model are in good agreement with laboratory measurements reported in the literature. When the model is applied to industrial incinerator conditions, at maximum a PCDD yield of 10(-3) microg/N m3 is calculated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
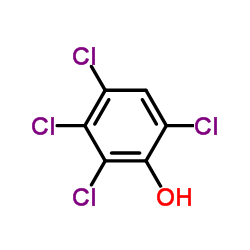 |
2,3,4,6-TETRACHLOROPHENOL
CAS:58-90-2 |
C6H2Cl4O |
|
PCDD/F formation from chlorophenols by lignin and manganese ...
2014-09-01 [Chemosphere 110 , 129-35, (2014)] |
|
Comparison of stir bar sorptive extraction and solid-phase m...
2008-05-15 [Talanta 75(3) , 753-9, (2008)] |
|
In situ polychlorophenol bioremediation potential of the ind...
2001-07-01 [Water Res. 35(10) , 2496-504, (2001)] |
|
Metabolism of Halophenols by 2,4,5-trichlorophenoxyacetic ac...
1983-11-01 [Appl. Environ. Microbiol. 46(5) , 1176-81, (1983)] |
|
Enhanced solubilization of arsenic and 2,3,4,6 tetrachloroph...
2004-10-01 [Chemosphere 57(3) , 197-206, (2004)] |