Determination of diphenylpyraline in plasma and urine by high-performance liquid chromatography.
K O Ebete, J E Koundourellis
Index: J. Chromatogr. B, Biomed. Appl. 677(2) , 319-23, (1996)
Full Text: HTML
Abstract
A rapid, reliable and specific reversed-phase high-performance liquid chromatographic procedure is described for the determination of diphenylpyraline hydrochloride at nanogram concentrations in plasma and urine. After extraction of the drug with n-pentane-2-propanol (50:1) from alkalinized samples, the organic extract was evaporated to dryness, reconstituted with methanol and chromatographed using a 5-micron Asahipak ODP-50 C18 column with UV detection at 254 nm. The elution time for diphenylpyraline was 7.9 min. The overall recovery of diphenylpyraline from spiked plasma and urine samples at concentrations ranging from 53 to 740 ng/ml were 94.65% and 92.29%, respectively. Linearity and precision data for plasma and urine standards after extraction were acceptable. The limit of detection was 15 ng/ml for both plasma and urine samples at 0.002 AUFS.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
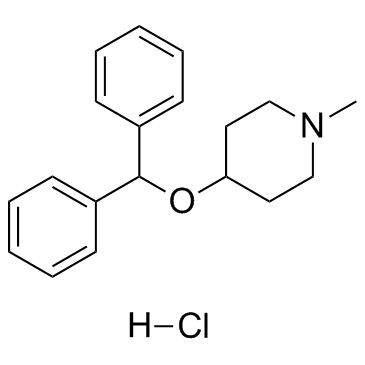 |
Diphenylpyraline (hydrochloride)
CAS:132-18-3 |
C19H24ClNO |
|
A possible approach to the suppression of side effects induc...
1995-01-01 [Prostaglandins Leukot. Essent. Fatty Acids 52(1) , 17-20, (1995)] |
|
Simultaneous determination of ten antihistamine drugs in hum...
2006-01-01 [Rapid Commun. Mass Spectrom. 20(4) , 537-43, (2006)] |
|
Effects of the histamine H₁ receptor antagonist and benztrop...
2012-05-15 [Eur. J. Pharmacol. 683(1-3) , 161-5, (2012)] |
|
Effects of H1 antihistamines on canine nasal vascular and ai...
1987-06-01 [Rhinology 25(2) , 95-100, (1987)] |
|
[Parkinsonism associated with cerebrotendinous xanthomatosis...
1990-09-01 [Rinsho. Shinkeigaku 30(9) , 978-84, (1990)] |