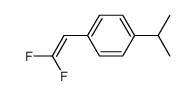
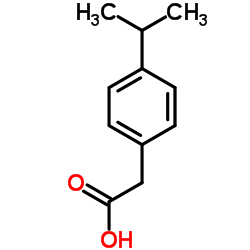
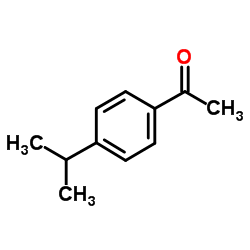
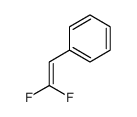
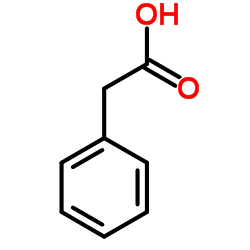
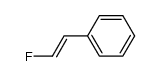
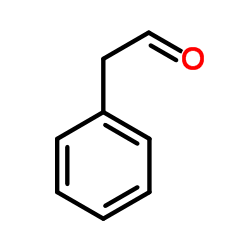
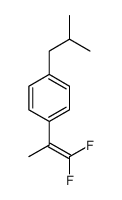
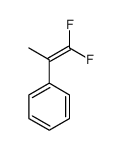
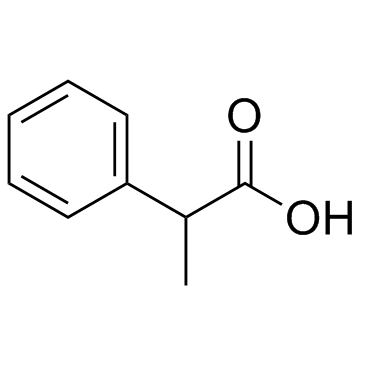
|
~69% |
|
~% |
|
~90% |
|
~% |
|
~79% |
|
~95% |
|
~78% |