Synthesis of a novel histidine analogue and its efficient incorporation into a protein in vivo.
Yutaka Ikeda, Shun-ichi Kawahara, Masumi Taki, Atsushi Kuno, Tsunemi Hasegawa, Kazunari Taira
Index: Protein Eng. 16 , 699-706, (2003)
Full Text: HTML
Abstract
Proteins containing unnatural amino acids have immense potential in biotechnology and medicine. We prepared several histidine analogues including a novel histidine analogue, beta-(1,2,3-triazol-4-yl)-DL-alanine. These histidine analogues were assayed for translational activity in histidine-auxotrophic Escherichia coli strain UTH780. We observed that several histidine analogues, including our novel histidine analogue, were efficiently incorporated into the protein in vivo; however, other analogues were rejected. These results suggest that the hydrogen atom at a specific position seriously affects incorporation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
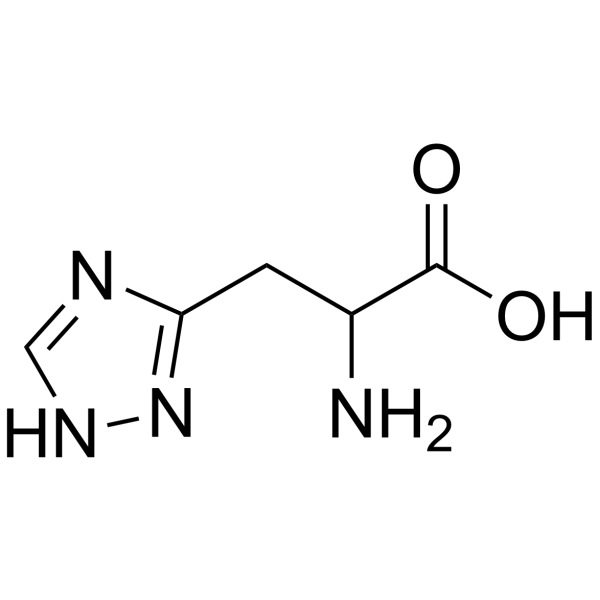 |
1H-1,2,4-Triazole-5-propanoicacid, a-amino
CAS:10109-05-4 |
C5H8N4O2 |
|
Incorporation of amino acid analogs during the biosynthesis ...
1980-09-09 [Biochim. Biophys. Acta 615(1) , 59-69, (1980)] |
|
Classifying mutagens as to their specificity in causing the ...
1986-01-01 [Environ. Mutagen. 8 , 9, (1986)] |
|
Simple and efficient synthesis of racemic 2-(tert-butoxycarb...
2011-01-01 [Molecules 16 , 3380-3390, (2011)] |
|
Incorporation of an unnatural amino acid in the active site ...
1996-04-01 [Protein Eng. 9(4) , 345-52, (1996)] |
|
Incorporation of 1,2,4-triazole-3-alanine into a mutant of p...
1998-03-01 [Protein Eng. 11(3) , 213-7, (1998)] |