Cytochrome P450BM-3 (CYP102): regiospecificity of oxidation of omega-unsaturated fatty acids and mechanism-based inactivation.
N Shirane, Z Sui, J A Peterson, P R Ortiz de Montellano
Index: Biochemistry 32(49) , 13732-41, (1993)
Full Text: HTML
Abstract
Cytochrome P450BM-3 preferentially oxidized fatty acids with terminal double or triple bonds to the omega-2 hydroxylated fatty acids rather than, respectively, to the epoxide or diacid metabolites. The enzyme is inactivated during catalytic turnover of long, terminally unsaturated fatty acids but not by the analogous medium-length fatty acids. Enzyme inactivation by 17-octadecynoic acid and 16-hydroxy-17-octadecynoic acid is due to alkylation of the prosthetic heme group to given an adduct tentatively identified as N-(2-oxo-3-hydroxy-17-carboxyheptadecyl)protoporphyrin IX by its chromatographic and spectroscopic properties. Catalytic turnover of 17-octadecenoic acid also results in heme modification. Fatty diacid monoethyl thioesters are introduced as a new class of irreversible inhibitors that exploit the omega-2 oxidation specificity of cytochrome P450BM-3. Catalytic oxidation of the monoethyl thioesters of dodecanedioic and hexadecanedioic acids results in enzyme inactivation and formation of the parent diacids as metabolites. Limited tryptic digestion of the enzyme after incubation with the monoethyl thioester of [14C]hexadecanedioic acid shows that the inactivating agent binds covalently to both the heme and flavin domains. This finding, and the observation that glutathione prevents inactivation of the enzyme by the monoethyl thioesters, indicate that a diffusible metabolite, probably the sulfoxide, is responsible for enzyme inactivation. The strong preference for omega-2 allylic or propargylic hydroxylation over terminal pi-bond oxidation is opposite to the usual cytochrome P450 pattern and requires that the enzyme actively suppress terminal pi-bond oxidation. The inference that the enzyme binds and sequesters the terminal carbon in a lipophilic pocket is consistent with the crystal structure of the hemoprotein domain of cytochrome P450BM-3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
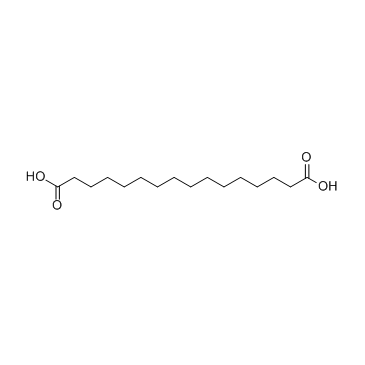 |
Hexadecanedioic acid
CAS:505-54-4 |
C16H30O4 |
|
Feasibility of detecting prostate cancer by ultraperformance...
2014-07-03 [J. Proteome Res. 13(7) , 3444-54, (2014)] |
|
Alkylthioacetic acids (3-thia fatty acids) as non-beta-oxidi...
1989-11-01 [J. Lipid Res. 30(11) , 1711-8, (1989)] |
|
Subcellular localization of hexadecanedioic acid activation ...
1974-11-01 [J. Lipid Res. 15(6) , 551-6, (1974)] |
|
Alkyl and carboxylalkyl esters of 4'-demethyl-4-deoxypodophy...
2004-02-01 [Eur. J. Med. Chem. 39(2) , 189-93, (2004)] |
|
Hexadecanedionic acid-sepharose 4B: A new tool for preparati...
2006-12-01 [J. Proteome Res. 5(12) , 3453-8, (2006)] |