| Structure | Name/CAS No. | Articles |
|---|---|---|
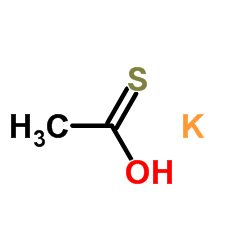 |
Potassium thioacetate
CAS:10387-40-3 |
|
 |
Thioacetic acid
CAS:507-09-5 |
|
 |
Sulconazole mononitrate
CAS:61318-91-0 |