| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Cefpodoxime Proxetil
CAS:87239-81-4 |
|
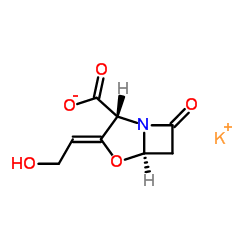 |
Clavulanate potassium
CAS:61177-45-5 |