| Structure | Name/CAS No. | Articles |
|---|---|---|
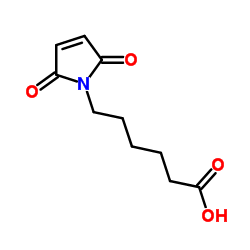 |
6-Maleimidocapronic acid
CAS:55750-53-3 |
|
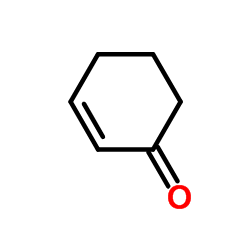 |
cyclohexenone
CAS:930-68-7 |