Application of Azure C for the extractive spectrophotometric determination of microgram amounts of penicillin.
A T Gowda, N M Gowda, H S Gowda, K S Rangappa
Index: J. Pharmacol. Methods 13(3) , 275-80, (1985)
Full Text: HTML
Abstract
A simple, accurate, and rapid method for the quantitative determination of penicillin is proposed. The method is based on the formation of a blue penicillin-Azure-C ion-pair that can be extracted into chloroform in phosphate-citric acid buffer. The molar absorptivities for sodium penicillin G and potassium penicillin V at 635 nm were 5.46 X 10(3) and 2.19 X 10(4) l/mol/cm, respectively. Beer's law was valid over the concentration range of 4-80 micrograms/ml for sodium penicillin G and 3-55 micrograms/ml for potassium penicillin v. Maximum absorbance was obtained almost instantaneously and was stable for several days. The method was successfully applied to pharmaceutical preparations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
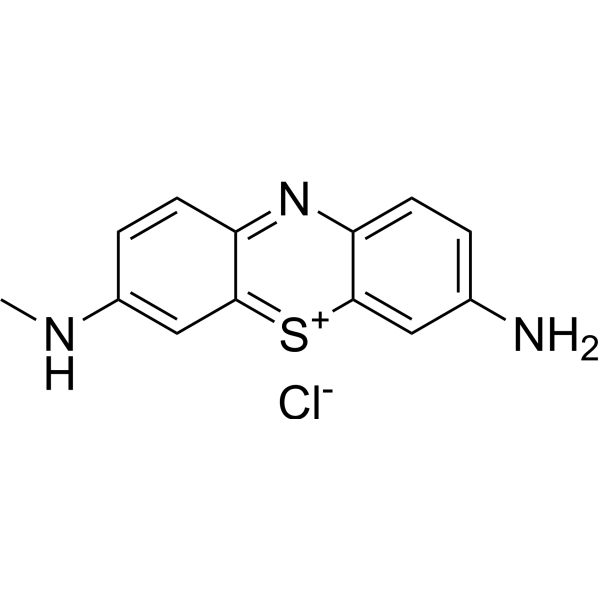 |
3-Amino-7-(methylamino)phenothiazin-5-ium chloride
CAS:531-57-7 |
C13H12ClN3S |
|
A chemical screening approach reveals that indole fluorescen...
2009-09-01 [Bioorg. Med. Chem. Lett. 19 , 4952-7, (2009)] |
|
Small molecule inhibitors of aggregation indicate that amylo...
2007-04-06 [J. Biol. Chem. 282 , 10311-24, (2007)] |
|
Note from the Biological Stain Commission: revision of azure...
1988-01-01 [Stain Technol. 63(1) , 65-9, (1988)] |
|
Electrochemical sensing based on redox mediation at carbon n...
2005-07-01 [Anal. Chem. 77(13) , 3960-5, (2005)] |
|
Chemical manipulation of hsp70 ATPase activity regulates tau...
2009-09-30 [J. Neurosci. 39th ed., 30;29 , 12079-12088, (2009)] |