| Structure | Name/CAS No. | Articles |
|---|---|---|
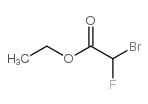 |
Ethyl bromofluoroacetate
CAS:401-55-8 |
Thibault Ferrary, Emilie David, Gaëlle Milanole, Tatiana Besset, Philippe Jubault, Xavier Pannecoucke
Index: Org. Lett. 15(21) , 5598-601, (2013)
Full Text: HTML
The synthesis of highly functionalized monofluorinated cyclopropanes based on a Michael Initiated Ring Closure (MIRC) reaction has been developed. The addition of quaternary ammonium salts derived from ethyl bromofluoroacetate on a panel of electron deficient alkenes followed by cyclization gave rise to an efficient access to monofluorinated cyclopropanes with good yields and remarkable diastereoselectivity.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Ethyl bromofluoroacetate
CAS:401-55-8 |
C4H6BrFO2 |
|
Visible-light-mediated fluoroalkylation of isocyanides with ...
2014-06-06 [Org. Lett. 16(11) , 2938-41, (2014)] |
|
Catalyzed Reformatsky reactions with ethyl bromofluoroacetat...
2002-01-11 [J. Org. Chem. 67(1) , 72-8, (2002)] |
|
[J. Fluor. Chem. 77 , 45, (1996)] |
|
[J. Chem. Soc. Perkin Trans. I , 49, (1991)] |
|
Synthesis of (E)-and (Z)-fluoro-olefin analogu...
[Tetrahedron Lett. 44(33) , 6231-34, (2003)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved