Synthesis and SAR of succinamide peptidomimetic inhibitors of cathepsin S.
Arnab K Chatterjee, Hong Liu, David C Tully, Jianhua Guo, Robert Epple, Ross Russo, Jennifer Williams, Michael Roberts, Tove Tuntland, Jonathan Chang, Perry Gordon, Thomas Hollenbeck, Christine Tumanut, Jun Li, Jennifer L Harris
Index: Bioorg. Med. Chem. Lett. 17(10) , 2899-903, (2007)
Full Text: HTML
Abstract
Peptidic, non-covalent inhibitors of lysosomal cysteine protease cathepsin S (1 and 2) were investigated due to low oral bioavailability, leading to an improved series of peptidomimetic inhibitors. Utilizing phenyl succinamides as the P2 residue increased the oral exposure of this lead series of compounds, while retaining selective inhibition of the cathepsin S isoform. Concurrent investigation of the P1 and P2 subsites resulted in the discovery of several potent and selective inhibitors of cathepsin S with good pharmacokinetic properties due to the elimination of saturated aliphatic P2 residues.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
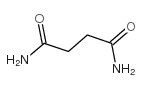 |
Butanediamide
CAS:110-14-5 |
C4H8N2O2 |
|
Effects of structural analogues of the substrate and alloste...
2009-08-01 [Bioorg. Med. Chem. 17 , 5414-9, (2009)] |
|
Synthesis of novel melanocortin 4 receptor agonists and anta...
2004-01-19 [Bioorg. Med. Chem. Lett. 14 , 377, (2004)] |
|
A disubstituted succinamide is a potent sodium channel block...
2004-08-03 [Biochemistry 43(30) , 9866-76, (2004)] |
|
Structure analysis reveals the flexibility of the ADAMTS-5 a...
2011-04-01 [Protein Sci. 20(4) , 735-44, (2011)] |
|
Adaptable synthesis of C-glycosidic multivalent carbohydrate...
2010-11-19 [Org. Lett. 12(22) , 5262-5, (2010)] |