Organic Letters
2010-11-19
Adaptable synthesis of C-glycosidic multivalent carbohydrates and succinamide-linked derivatization.
Gavin J Miller, John M Gardiner
Index: Org. Lett. 12(22) , 5262-5, (2010)
Full Text: HTML
Abstract
A modular approach to the synthesis of trivalent C-glycosidic carbohydrates is described. The approach is illustrated employing carboxylate-terminated C-glycosidic d-mannose, d-glucose, and d-galactose derivatives with different length C1-linked spacer units and also core units with different length linker units attached. The central core scaffold is additionally functionalized via a succinamide-based, conjugatable linker unit, exemplified in an extended multivalent derivative [31] and a pyrene-bearing fluorsecent-labeled tris-C-mannosyl conjugate [33].
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
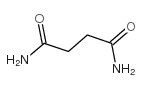 |
Butanediamide
CAS:110-14-5 |
C4H8N2O2 |
Related Articles:
More...
|
Effects of structural analogues of the substrate and alloste...
2009-08-01 [Bioorg. Med. Chem. 17 , 5414-9, (2009)] |
|
Synthesis of novel melanocortin 4 receptor agonists and anta...
2004-01-19 [Bioorg. Med. Chem. Lett. 14 , 377, (2004)] |
|
A disubstituted succinamide is a potent sodium channel block...
2004-08-03 [Biochemistry 43(30) , 9866-76, (2004)] |
|
Structure analysis reveals the flexibility of the ADAMTS-5 a...
2011-04-01 [Protein Sci. 20(4) , 735-44, (2011)] |
|
Synthesis of novel succinamide derivatives having the 5,11-d...
1997-06-01 [Chem. Pharm. Bull. 45(6) , 996-1007, (1997)] |