Synthesis of novel succinamide derivatives having the 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one skeleton as potent and selective M2 muscarinic receptor antagonists. I.
T Watanabe, I Kinoyama, A Kakefuda, T Okazaki, K Takizawa, S Hirano, H Shibata, I Yanagisawa
Index: Chem. Pharm. Bull. 45(6) , 996-1007, (1997)
Full Text: HTML
Abstract
A series of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one derivatives containing the succinamide skeleton has been synthesized and evaluated for M1, M2 and M3 muscarinic receptor binding affinities (in vitro) and M2 and M3 muscarinic receptor antagonistic activities (in vivo). Some of them showed higher and more selective binding affinities for M(2) muscarinic receptors than that of AF-DX 116. Among them, 11-[3-[N-[2-(N-benzyl-N- methylamino)ethyl]-N-ethylcarbamoyl]propionyl]-5,11-dihydro-6H-pyr ido [2,3-b][1,4]benzodiazepin-6-one (68) was found to be the most potent and selective M2 muscarinic receptor antagonist in vitro. This compound also strongly inhibited the oxotremorine-induced bradycardia after intravenous administration and showed 130-fold selectivity for M2 muscarinic receptors over M3 muscarinic receptors in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
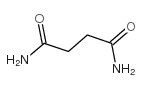 |
Butanediamide
CAS:110-14-5 |
C4H8N2O2 |
|
Effects of structural analogues of the substrate and alloste...
2009-08-01 [Bioorg. Med. Chem. 17 , 5414-9, (2009)] |
|
Synthesis of novel melanocortin 4 receptor agonists and anta...
2004-01-19 [Bioorg. Med. Chem. Lett. 14 , 377, (2004)] |
|
A disubstituted succinamide is a potent sodium channel block...
2004-08-03 [Biochemistry 43(30) , 9866-76, (2004)] |
|
Structure analysis reveals the flexibility of the ADAMTS-5 a...
2011-04-01 [Protein Sci. 20(4) , 735-44, (2011)] |
|
Adaptable synthesis of C-glycosidic multivalent carbohydrate...
2010-11-19 [Org. Lett. 12(22) , 5262-5, (2010)] |