Novel P2-P4 macrocyclic inhibitors of HCV NS3/4A protease by P3 succinamide fragment depeptidization strategy.
Marco Pompei, Maria Emilia Di Francesco, Silvia Pesci, Uwe Koch, Sue Ellen Vignetti, Maria Veneziano, Paola Pace, Vincenzo Summa
Index: Bioorg. Med. Chem. Lett. 20(1) , 168-74, (2010)
Full Text: HTML
Abstract
Hepatitis C represents a serious worldwide health-care problem. Recently, we have disclosed a novel class of P2-P4 macrocyclic inhibitors of NS3/4A protease containing a carbamate functionality as capping group at the P3 N-terminus. Herein we report our work aimed at further depeptidizing the P3 region by replacement of the urethane function with a succinamide motif. This peptidomimetic approach has led to the discovery of novel P2-P4 macrocyclic inhibitors of HCV NS3/4A protease with sub-nanomolar enzyme affinities. In addition to being potent inhibitors of HCV subgenomic replication, optimized analogues within this series have also presented attractive PK properties and showed promising liver levels in rat following oral administration.Copyright 2009 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
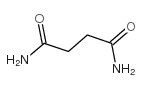 |
Butanediamide
CAS:110-14-5 |
C4H8N2O2 |
|
Effects of structural analogues of the substrate and alloste...
2009-08-01 [Bioorg. Med. Chem. 17 , 5414-9, (2009)] |
|
Synthesis of novel melanocortin 4 receptor agonists and anta...
2004-01-19 [Bioorg. Med. Chem. Lett. 14 , 377, (2004)] |
|
A disubstituted succinamide is a potent sodium channel block...
2004-08-03 [Biochemistry 43(30) , 9866-76, (2004)] |
|
Structure analysis reveals the flexibility of the ADAMTS-5 a...
2011-04-01 [Protein Sci. 20(4) , 735-44, (2011)] |
|
Adaptable synthesis of C-glycosidic multivalent carbohydrate...
2010-11-19 [Org. Lett. 12(22) , 5262-5, (2010)] |