European Journal of Medicinal Chemistry
2008-01-01
Synthesis of N-(5,7-diamino-3-phenyl-quinoxalin-2-yl)-3,4,5-substituted anilines and N-[4[(5,7-diamino-3-phenylquinoxalin-2-yl)amino]benzoyl]-l-glutamic acid diethyl ester: evaluation of in vitro anti-cancer and anti-folate activities.
Paola Corona, Mario Loriga, M Paola Costi, Stefania Ferrari, Giuseppe Paglietti
Index: Eur. J. Med. Chem. 43(1) , 189-203, (2008)
Full Text: HTML
Abstract
Several diamino quinoxalines were designed, synthesized and evaluated as anti-tumor agents. Two compounds showed the most potent cytotoxic activities against the leukemia CCRF-CEM cell line (GI(50)<0.01microM) and the ovarian cancer cell line OVCAR-4 (GI(50)=0.03microM), respectively, with comparable/better activities than Methotrexate (MTX). Docking calculations of the complexes of hDHFR with the most active compounds identified the binding mode of the described molecules with respect to MTX.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
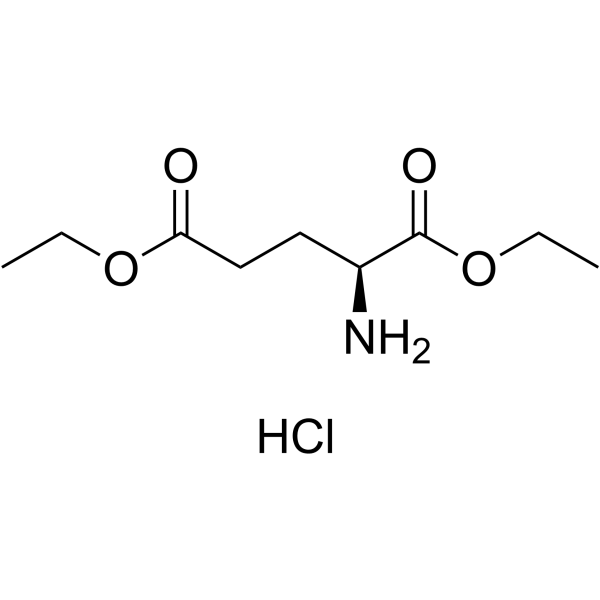 |
H-Glu(OEt)-OEt.HCl
CAS:1118-89-4 |
C9H18ClNO4 |
Related Articles:
More...
|
Fast-acting excitatory amino acids are involved in the enhan...
2002-11-08 [Life Sci. 71(25) , 2961-72, (2002)] |
|
Glycine increases arterial pressure and augments NMDA-induce...
1997-12-11 [J. Auton. Nerv. Syst. 67(3) , 145-55, (1997)] |
|
Mass spectrometric and kinetic studies on slow progression o...
2008-06-01 [Biochim. Biophys. Acta 1780(6) , 881-91, (2008)] |
|
Neurotransmitters in the thalamus relaying visceral input to...
2001-11-01 [Am. J. Physiol. Regul. Integr. Comp. Physiol. 281(5) , R1665-74, (2001)] |
|
Neonatal glutamate can destroy the hippocampal CA1 structure...
1993-07-09 [Brain Res. 616(1-2) , 311-4, (1993)] |